
Supporting a positive
patient experience…
Omnipaque for Oral Use
Approved for use in adults and children1
A neutral taste for your patients
- Shown to have a neutral taste when compared with ionic Gastrografin® (diatrizoate meglumine and diatrizoate sodium solution USP)2
- Omnipaque received a significantly better taste preference score than did MD-Gastroview® (diatrizoate meglumine and diatrizoate sodium solution USP) (P<0.001)3
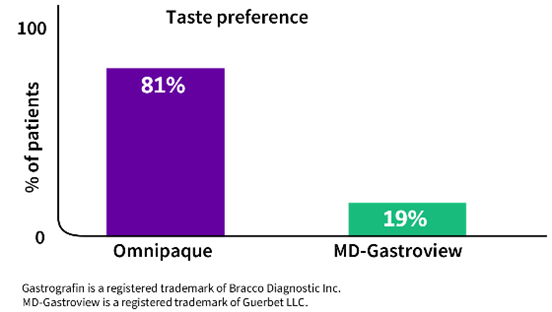
Adults and kids will drink it!4
- A nonionic, low-osmolar contrast medium indicated for oral use in both adults and children1
- Shown to have a neutral taste when compared with ionic diatrizoate2
- In a study of pediatric patients by Smevik and Westvik, 98% of children drank the entire dose5
- Patients in an abdominal computerized tomography study drank the entire prescribed amount and said they would do so again, if necessary6
- Well suited for gastrointestinal (GI) use in infants and children7
Complete the form on the right to download the visual aid from GE HealthCare and learn more about the potential benefits of Omnipaque for Oral Use – both for you and your patients.
IMPORTANT SAFETY INFORMATION ABOUT OMNIPAQUE™ (iohexol)
|
WARNING: RISKS WITH INADVERTENT INTRATHECAL ADMINISTRATION OF Omnipaque Injections 140 and 350 mgI/mL
Inadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema. |
CONTRAINDICATIONS:
- Omnipaque 140 and Omnipaque 350 are contraindicated for intrathecal use.
- Omnipaque Oral Solutions 9 and 12 are contraindicated for parenteral administration.
- Omnipaque body cavity 240 and 300 for hysterosalpingography is contraindicated during pregnancy (or suspected pregnancy), menstruation (or when menstruation is imminent), within 6 months after termination of pregnancy, within 30 days after conization or curettage, when signs of infection are present in any portion of the genital tract, including the external genitalia, and when reproductive tract neoplasia is known or suspected.
ADVERSE REACTIONS:
- Intrathecal: Headaches, pain including backache, neckache, stiffness and neuralgia, nausea, vomiting, dizziness.
- Intravascular: Pain, vision abnormalities, (including blurred vision and photomas), headache, taste perversion, arrhythmias including premature ventricular contractions (PVCs) and premature atrial contractions (PACs), angina/chest pain, nausea.
- Oral: Diarrhea, nausea, vomiting, abdominal pain, flatulence, headache.
- Body cavity: Pain, swelling, heat sensation.
- Postmarketing adverse events seen include: Hypersensitivity and manifestations such as rash, pruritus, urticaria and dyspnea, chest pain, swelling.
Please see additional Important Safety Information and Full Prescribing Information for Omnipaque, including Boxed Warning, here.
References
- Omnipaque [prescribing information]. Marlborough, MA: GE HealthCare; 2022.
- Stordahl A, Laerum F, Gjølberg T, Enge I. Water-soluble contrast media in radiography of small bowel obstruction: comparison of ionic and non-ionic contrast media. Acta Radiol. 1988;29:53-56.
- McNamara MM, Lockhart ME, Fineberg NS, Berland LL. Oral contrast media for body ct: comparison of diatrizoate sodium and iohexol for patient acceptance and bowel opacification. AJR. 2010;195:1137-1141.
- Smevik B, Stake G. Omnipaque as a contrast medium for bowel opacification in abdominal CT in infants and children. In: Kaufmann HJ, ed. Contrast Media in Child Radiology. Basel, Switzerland: Karger; 1986:79-80.
- Smevik B, Westvik J. Iohexol for contrast enhancement of bowel in pediatric abdominal CT. Acta Radiol. 1990;31:601-604.
- Lönnemark M, Magnusson A. Oral contrast media in CT of the abdomen: iohexol of different concentrations as a gastrointestinal contrast medium. Acta Radiol. 1995;36:396-398.
- Stake G, Smevik B. Iohexol as contrast medium for the gastrointestinal tract in childhood. In: Kaufmann HJ, ed. Contrast Media in Child Radiology. Basel, Switzerland: Karger; 1986:107-109.
Download Now
GE HealthCare | Privacy Policy | Terms of Use | Contact Us | Unsubscribe
500 W. Monroe Street | Chicago, IL 60661
© 2023 GE HealthCare
GE is a trademark of General Electric Company used under trademark license.
April 2023 JB08294US