
Helping clinicians detect whole-body ER+ lesion status to better inform clinical decisions ¹
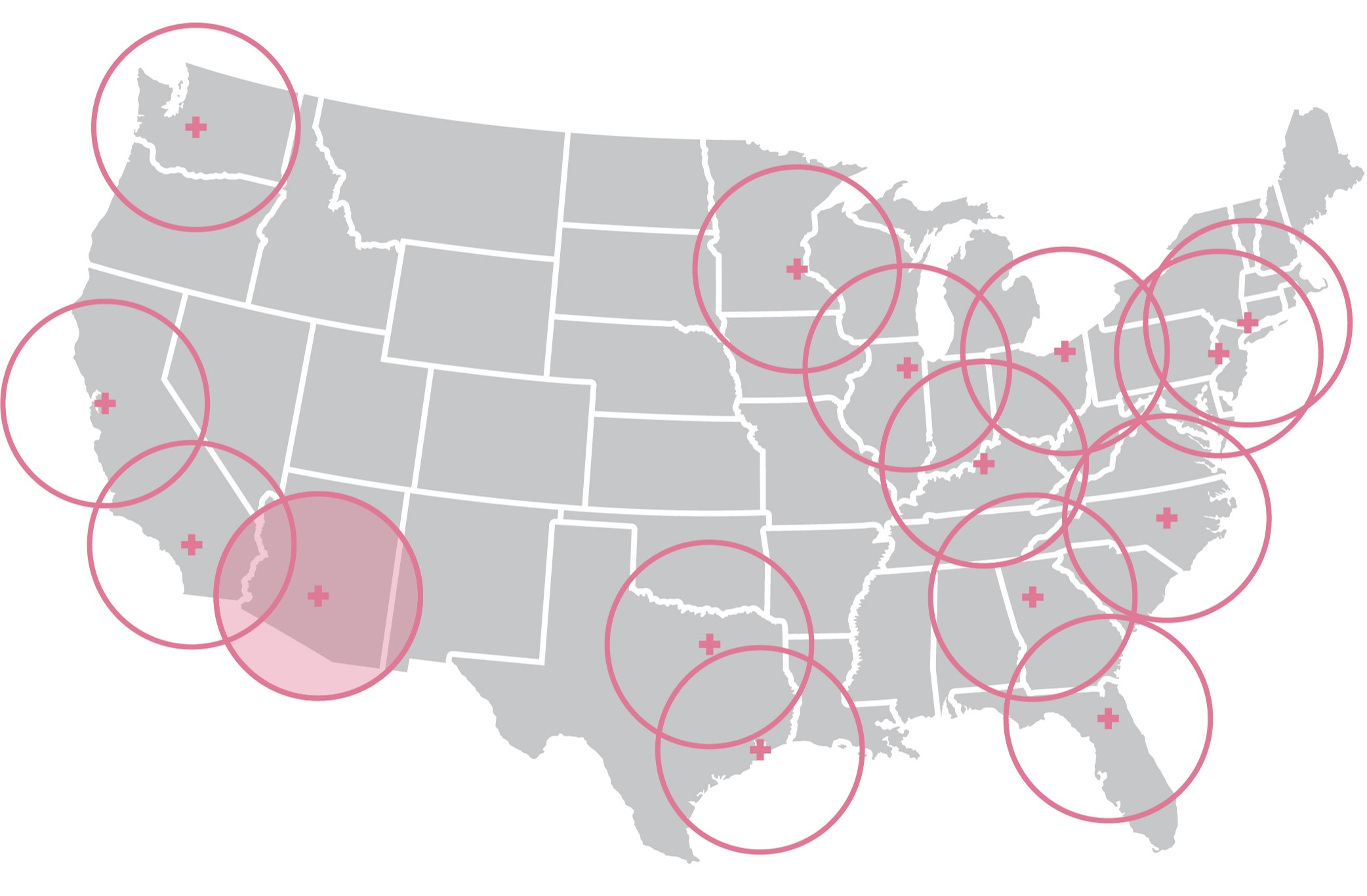
Cerianna (F18 fluoroestradiol) is now available within a 3-hour driving radius from PETNET centers in the Phoenix region.
Thank you for your interest in learning more about ER discordance and the role of Cerianna™ (fluoroestradiol F 18) Injection in metastatic breast cancer. Click the download button to learn more about:
- The role of estrogen receptors (ERs) in breast cancer heterogeneity, proliferation, and response to therapy
- The importance of ER expression and discordance in metastatic BC
- How clarifying the extent of within-patient ER discordance may be critically important for clinical decision making1,2
Request information on how Cerianna may help identify ER discordance and impact clinical decisions in your patients with recurrent or metastatic breast cancer.
Watch Dr. Louis Mazzarelli present the webinar “Cerianna: Seeing Deeper into Reccurent or Metastatic Breast Cancer”
Speak with a representative
References: 1. Yang Z, et al. Clin Breast Cancer. 2013;13(5):359-363. 2. Kurland BF, et al. Oncologist. 2020;25(10):835-844.
INDICATIONS AND USAGE:
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use:
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
Important Safety Information
CONTRAINDICATIONS
- None.
WARNINGS AND PRECAUTIONS
Risk of Misdiagnosis
Inadequate Tumor Characterization and Other ER-Positive Pathology
- Breast cancer may be heterogeneous within patients and across time. CERIANNA images ER and is not useful for imaging other receptors such as HER2 and PR. The uptake of fluoroestradiol F 18 is not specific for breast cancer and may occur in a variety of ER-positive tumors that arise outside of the breast, including from the uterus and ovaries. Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer.
False Negative CERIANNA Scan
- A negative CERIANNA scan does not rule out ER-positive breast cancer. Pathology or clinical characteristics that suggest a patient may benefit from systemic hormone therapy should take precedence over a discordant negative CERIANNA scan.
Radiation Risks
- Diagnostic radiopharmaceuticals, including CERIANNA, expose patients to radiation. Radiation exposure is associated with a dose-dependent increased risk of cancer. Ensure safe drug handling and patient preparation procedures (including adequate hydration and voiding) to protect patients and health care providers from unintentional radiation exposure.
Pregnancy Status
- Assessment of pregnancy status is recommended in females of reproductive potential before administering CERIANNA.
ADVERSE REACTIONS
- In Clinical Trials (n=1207) the most common adverse reactions seen occurred at a rate < 1%: were injection-site pain and dysgeusia.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
- All radiopharmaceuticals, including CERIANNA, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of radiation dose. Advise a pregnant woman of the potential risks of fetal exposure to radiation from administration of CERIANNA.
- There are no available data on CERIANNA use in pregnant women. No animal reproduction studies using fluoroestradiol F 18 have been conducted to evaluate its effect on female reproduction and embryo-fetal development.
- The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Lactation
Risk Summary
- There are no data on the presence of fluoroestradiol F 18 in human milk, or its effects on the breastfed infant or milk production. Lactation studies have not been conducted in animals. Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration in order to minimize radiation exposure to a breastfed infant.
Pediatric Use
- The safety and effectiveness of CERIANNA in pediatric patients have not been established.
Geriatric Use
- Clinical studies of fluoroestradiol F 18 injection did not reveal any difference in pharmacokinetics or biodistribution in patients aged 65 and over.
DRUG INTERACTIONS
Systemic Endocrine Therapies that Target Estrogen Receptors
- Certain classes of systemic endocrine therapies, including ER modulators and ER down-regulators, block ER, reduce the uptake of fluoroestradiol F 18, and may reduce detection of ER-positive lesions after administration of CERIANNA. Drugs from these classes such as tamoxifen and fulvestrant may block ER for up to 8 and 28 weeks, respectively. Do not delay indicated therapy in order to administer CERIANNA. Administer CERIANNA prior to starting systemic endocrine therapies that block ER.
To report SUSPECTED ADVERSE REACTIONS, contact Zionexa US Corp, a GE Healthcare Company at +1.800.654.0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
GE Healthcare | Privacy Policy | Terms of Use | Contact Us | Unsubscribe
500 W. Monroe Street | Chicago, IL 60661
© 2022 General Electric Company
GE and the GE Monogram are trademarks of the General Electric Company.
JB06779US July/2022
