© 2019 General Electric Company – All rights reserved. GE and GE monogram are trademarks of General Electric Company.
GE Healthcare is a division of General Electric Company. TM. Trademark of General Electric Company.
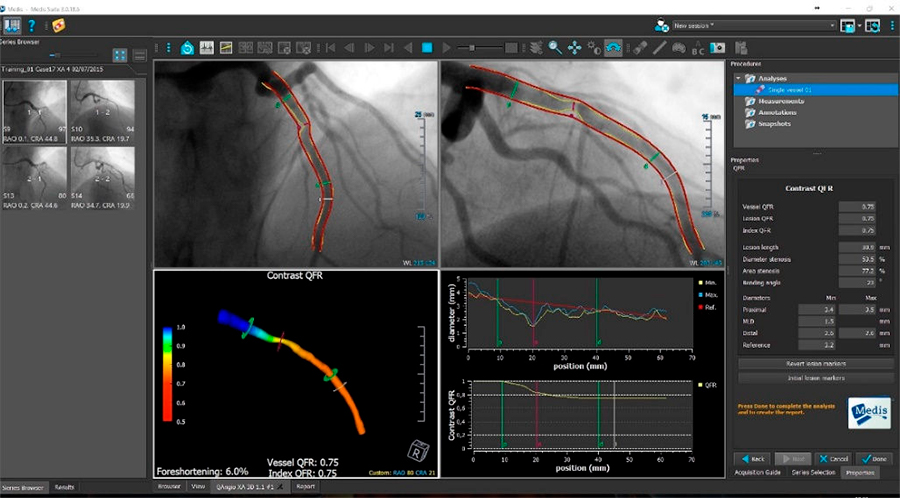
*GE IGS imaging systems refer to Innova IGS 5, Innova IGS 6, Discovery IGS 7 and Discovery IGS 7 ORQFR® Analysis is included in QAngio® XA 3D software. Sold by Medis medical imaging systems. It is applicable to Innova IGS 5, Innova IGS.
510(k) clearance for QAngio XA 3D is pending, not available for sale within the United States.
Innova IGS 520, Innova IGS 530, Innova IGS 540, Discovery IGS 730, discovery IGS 740 Intended use:Medical device, X-ray equipment for diagnostic, interventional and hybrid surgical procedures. Always refer to the complete User’s manual before use and carefully read all instructions to ensure the good use of your medical device. Last revision: 2014-11-24
Innova IGS 620, Innova IGS 630 Intended use: Medical device, X-ray equipment for diagnostic and interventional procedures.Class/Notified Body: IIb/ CE 0459. Manufacturer: GE MEDICAL SYSTEMS SCS. Always refer to the complete User’s manual before use and carefully read all instructions to ensure the good useof your medical device. Last revision: 2014-11-24