
Bile Acid Diarrhoea is common and is often misdiagnosed as IBS-D
Bile acid diarrhoea (BAD) is a common cause of chronic diarrhoea and is often misdiagnosed as IBS-diarrhoea. It is estimated that 1% of the population are affected by BAD2.
More specifically 1 in 3 patients (33%–45%) with a diagnosis of functional diarrhoea or IBS-D may have a component of bile acid diarrhoea3,4.
These data suggest that type II bile acid malabsorption is the underlying, and potentially treatable, cause of symptoms in a significant proportion of individuals labelled as having either chronic functional diarrhoea or IBS-D5.
If you would like more information on BAD and its misdiagnosis, please contact us.
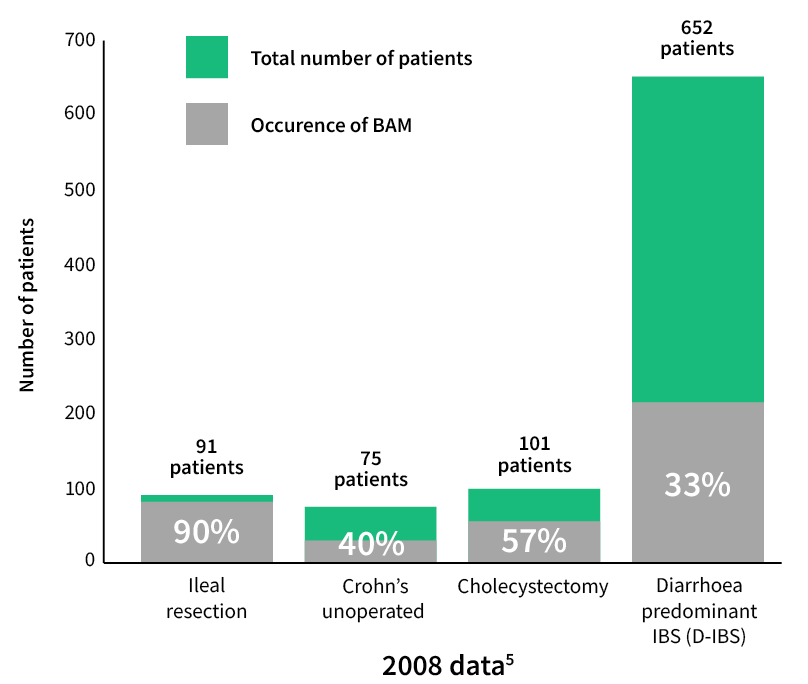
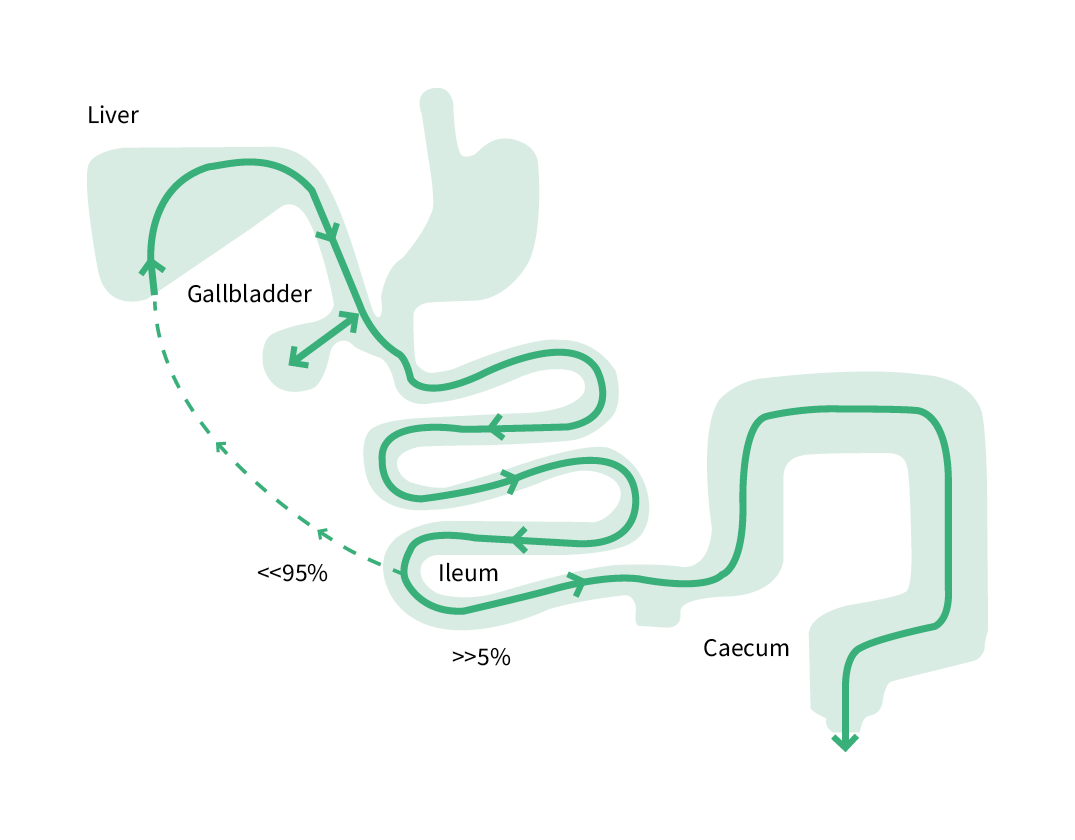
Accurate diagnosis is essential for management of BAD disease
It is recognised that BAD is a chronic condition requiring long term therapy to achieve a significant improvement in diarrhoea symptoms, and therefore warrants a clear diagnosis;6 The results of the diagnosis directly impact the choice of treatment for a person with BAD7 .
Early diagnosis of BAD is predicted to lead to decreased morbidity, increased quality of life and fewer referrals to gastroenterologists8.
Accurate diagnosis of BAD, strengthens the case for continuing with Bile Acid Sequestrants therapy which, could lead to improvements in symptoms and quality of life9 .
If you would like more information on the diagnosis of BAD, please contact us.
Learn more about the diagnosis of BAD in YOUR patients
If you want to learn more about the diagnosis of BAD in YOUR patients, please complete the form below and we will provide you with all the information needed
Product Information
Please see SeHCATTM product information here for:
Sweden | Finland | Norway | Netherlands | BELUX FR | BELUX NL
Reporting Adverse Events
GE Healthcare has a legal and ethical responsibility to protect the public health and therefore we ensure safety and efficiency for our products.
In order to do so, we collect reports about suspected adverse events that the patients experience during or after they are exposed to our products.
We also collect information about special situations (such as exposure during pregnancy, overdose, etc. ) as well as customer complaints and other views from customers about our products.
To report a suspected adverse event/special situation, customer complaint or just to share your views about our products, please contact:
For Sweden: [email protected] | Phone: 0200 895473
For Finland: [email protected] | Phone: 0800 919 427
For Norway: [email protected] | Phone: 23 18 79 58
For Netherlands: [email protected] | Phone: 31 40 299 1111
For BELUX: C[email protected] or [email protected] | Phone: France 32 80 04 96 18 | Netherlands 32 80 03 89 97
References:
- Wedlake L et al. Aliment Pharmacol Ther 2009; 30:707-717
- Gupta A et al. Support Care Cancer 2015; 23:2881-2890
- Mottacki N et al. Alliment Pharmacol Ther 2016; 43 (8): 884-98.
- Fernández-Bañares F et al. Am J Gastroenterol 2007; 102: 2520-8
- Riemsma R et al. Health Technology Assessment 2013; 17:1-258
- Lin S, et al. European Journal of Gastroenterology & Hepatology 2016 pp.28(2)240-245
- Kumar L et al. Gut 2013 p.62:A284
- Bannaga A, et al. BMJ Open Gastroenterol 2016;3:e000116
- Smith MJ et al. J R Coll Physicians Lond 2000; 34 (5): 448-51
GE Healthcare | Privacy Policy | Terms of Use | Contact Us | Unsubscribe
GE Healthcare Limited, Pollards Wood, Nightingales Lane, Chalfont St Giles, Buckinghamshire, England HP8 4SP
© 2023 GE HealthCare
SeHCAT is a trademark of GE HealthCare.
GE is a trademark of General Electric Company used under trademark license.
JB00354BE | January 2023
.

Welcome to GE Healthcare
The content on this website is intended only for an audience of Healthcare Professionals located in Northern Europe. If you are from a country other than Northern Europe, please visit the GEHealthcare.com Global Gateway and select your country.
Are you a healthcare professional from Northern Europe (SE,FI,NO,NLD,BELUX)?
Yes No